The rapid design of catalysts is a key step towards widely accessible electric vehicles more efficient depollution catalysts, cleaner factories... Researchers have developed a simple and faster method to improve the efficiency of a family of catalysts. These compounds indispensable to the industry, facilitate chemical reactions.
This breakthrough is the result of a collaboration between theoretical chemists of ENS Lyon, CNRS, Université Claude Bernard Lyon 1 (France), Leiden University (the Netherlands), and experimental chemists of Ruhr-Universität Bochum and Technishe Universität München (Germany). Their findings are published in Science on October 9,2015
Catalysts are compounds that interact with the reactants of chemical reactions to minimize the energy required for the formation of the desired products. The European chemical industry, which largely relies on catalytic processes, generates an annual trade surplus of € 50 billion (Chlorine Industry Review 2012-2013. Euro Chlor, Belgium, 2013. URL: www.eurochlor.org). Apart from the large economic profits, catalysis-based devices such as exhaust gas converters help mitigate the environmental damage of internal-combustion engines. Besides, if only a small number of cars equipped with fuel cells are currently commercialized, despite the fact that they do not emit greenhouse gases, it is because the catalysts for these fuel cells are not efficient and durable enough. The development of efficient catalysts is, therefore, a major societal challenge.
Catalysts are often formed of small metallic particles with diameters in the range of a few nanometers. Their efficiency depends on their size, shape and chemical composition. Until now, in order to improve a catalyst, chemists only had one clue: the optimal interaction strength between the reactants and the catalyst. Owing to the lack of explicit relationships between this optimal interaction and the structures of catalyst nanoparticles, large databases had to be screened to find materials that possess such energetics, and experimenters needed to conduct numerous tests on the most active candidates.
Today, an international team of chemists has developed a new approach, which allows the determination of the optimal structure of the catalytic site (interaction site between the catalyst and the reactants). Their design procedure is based on a simple chemical concept: coordination numbers (see figure).
They have experimentally validated this approach by designing a new type of Pt-based catalysts for fuel cell applications. The optimal efficiency was predicted for sites with a coordination number higher than the reference catalyst surfaces. The new sites were created by digging atomic-size cavities into platinum surfaces. After having created these cavities on platinum surfaces using three different methods, the result was clear: the catalytic efficiency can be enhanced by up to 3.5 times.
This work should allow a decrease of the time needed to develop catalysts and opens the way for the design of efficient and commercially viable fuel cells, to a larger usage of hydrogen as a clean fuel and more generally, in the future, to the optimization of several industrial processes.
The research leading to these results received funding from The Netherlands Organisation for Scientific Research (NWO), Veni project 722.014.009; the European Union’s FP7/2007-2013 program, FCHJT initiative, grant 303419 (PUMA MIND); the Cluster of Excellence RESOLV (EXC 1069) funded by Deutsche Forschungsgemeinschaft, Helmholtz-Energie-Allianz (HAE-0002), and SFB 749; and the Cluster of Excellence Nanosystems Initiative Munich.
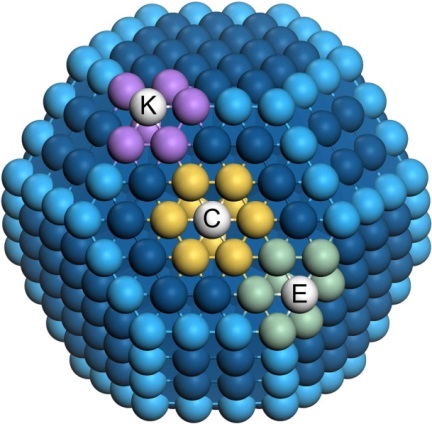
Catalytic nanoparticles containing various different sites. The coordination numbers are, for the site C (centre of a hexagonal facet) nine neighbours marked in yellow, for the site E (edge between two hexagonal facets) seven neighbours in green and for the site K (corner between three facets) six neighbours in purple. Atoms at edges and terraces appear in light and dark blue, respectively. Each site contributes differently to the total catalytic activity by virtue of its number of neighbours (first and second neighbours have actually to be considered).
© D. Loffreda – CNRS/ENS Lyon.
Reference:
(1) Finding optimal surface sites on heterogeneous catalysts by counting nearest neighbors, Federico Calle-Vallejo, Jakub Tymoczko, Viktor Colic, Quang Huy Vu, Marcus D. Pohl, Karina Morgenstern, David Loffreda, Philippe Sautet, Wolfgang Schuhmann, Aliaksandr S. Bandarenka. Science, 9 octobre 2015.
DOI : 10.1126/science.aab3501
France Scientist CNRS l Philippe Sautet l T 04 72 72 81 55 / 06 15 09 68 36 l philippe.sautet@ens-lyon.fr
Press CNRS l Véronique Etienne l T 01 44 96 51 37 l veronique.etienne@cnrs-dir.fr
Germany
The Netherlands: Federico Calle-Vallejo | +31 (0)71 527 4252 | f.calle.vallejo@chem.leidenuniv.nl



 en
en es
es

